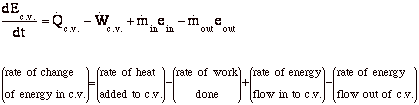Energy Balance Formula Thermodynamics

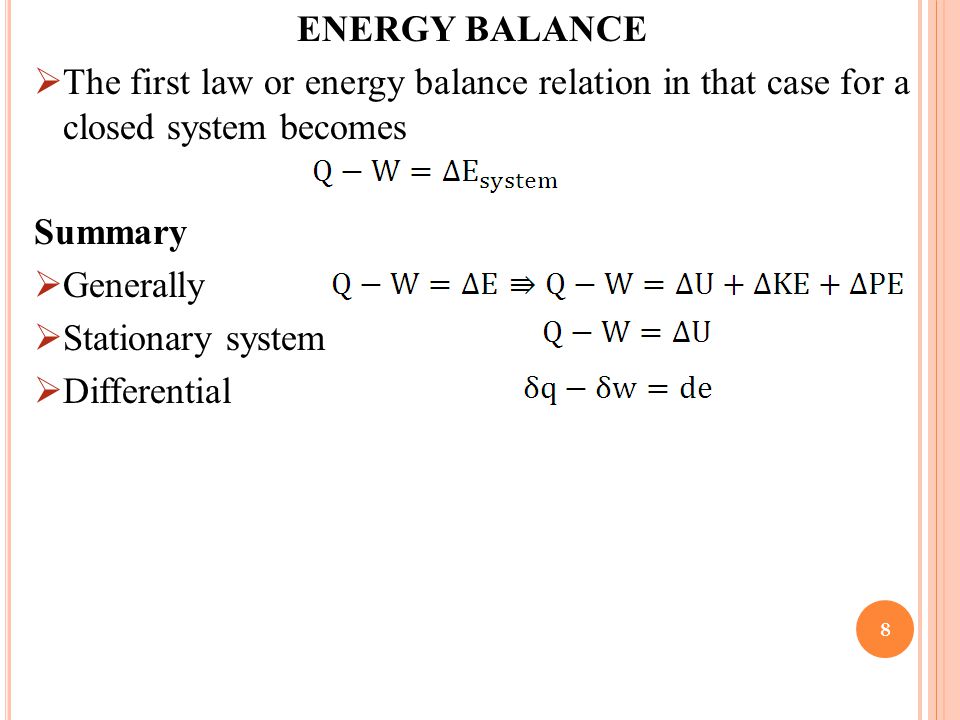
The first law of thermodynamics for the closed system in differential form is du dq dw if the potential and kinetic energy are ignorable.
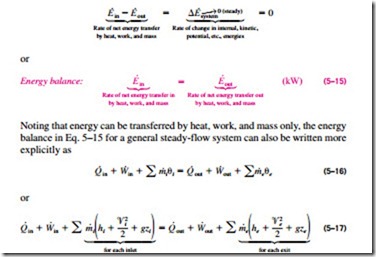
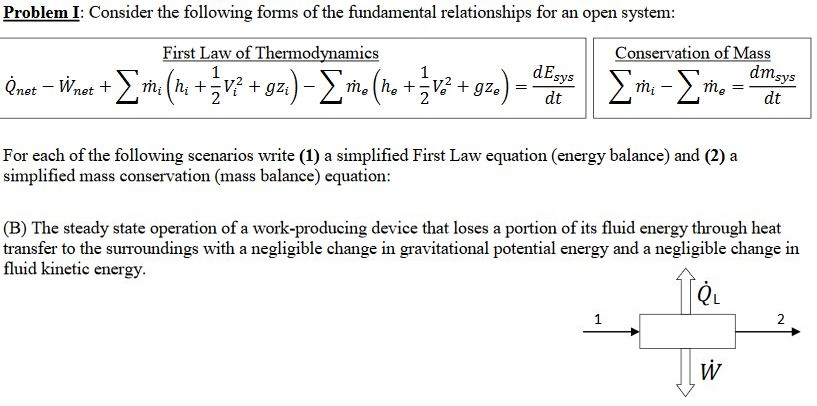
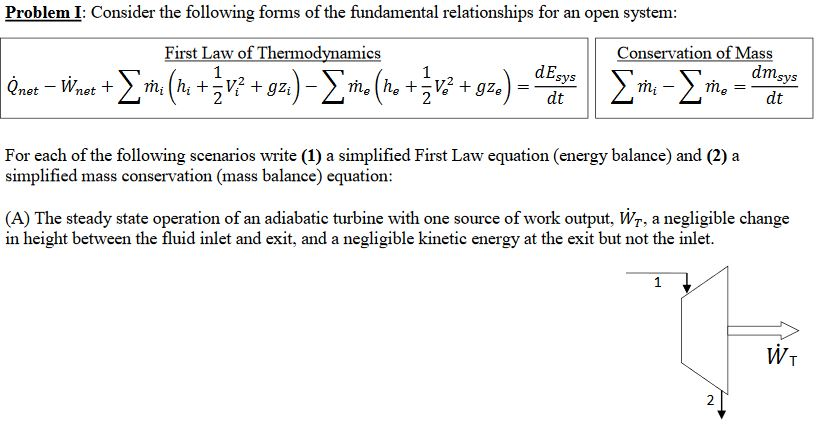
Energy balance formula thermodynamics. Above equation of mass balance for a steady flow process is also termed as equation of continuity. 𝑑𝐸𝐶𝐶 𝑑𝑑 𝑄 𝑊 𝑚. Accumulation of energy in system input of energy into system output of energy from system 6 now the total energy of a system as considered above is composed of kinetic potential and internal energies. Therefore the energy balance is.
The internal energy has changed from its level at instant 1 to another level at instant 2 after heat is applied. This form of energy is referred to as flow energy pν work. Entropy balance 2nd law. Let us implement here the assumptions made above in order to secure the energy equation for throttling devices in quite simple terms.
The internal energy u is a thermodynamic property. They may be combined into what is known as fundamental thermodynamic relation which describes all of the changes of thermodynamic state functions of a system of uniform temperature and pressure. The general energy balance for a process can be expressed in words as. The first and second law of thermodynamics are the most fundamental equations of thermodynamics.
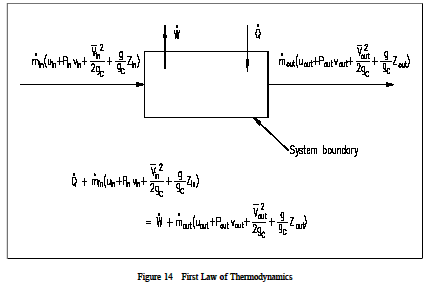
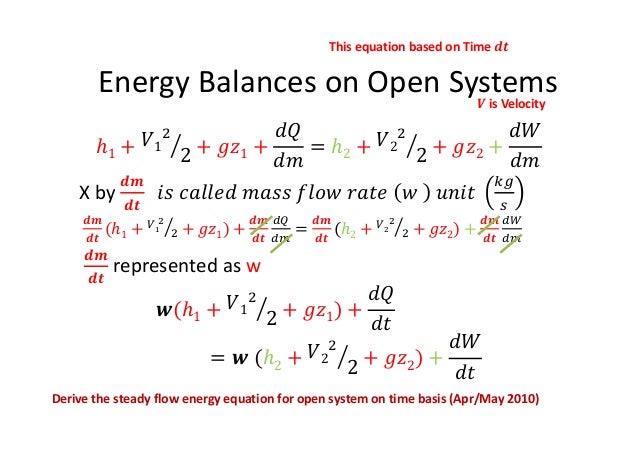
Where 𝑚 𝐴𝑉 𝜈 is the mass flow rate conservation of energy 1st law. Control volume mass flow across system boundaries conservation of mass. 𝑑𝑚𝐶𝐶 𝑑𝑑 𝑚 𝑖 𝑚𝑒. The thermodynamic terms thus representing the various forms of energy crossing the control boundary with the mass are given as m u pν ke pe.
Where v1 and a1 are the velocity and area of cross section of stream respectively at section 1 1 of control volume system. 𝑆 𝛿𝛿 𝑇 𝑏 𝜎. Then the first law equation becomes like this. Schmidt rohr 2014 as a simple example consider a system composed of a number of k different types of.
So the enthalpy represents the energy of a material in transit so it is the summation of internal energy and the flow work. Heat is the energy transfer due to temperature difference. H 1 h 2 where h 1 and h 2 are the enthalpy of the fluid at inlet and outlet respectively.